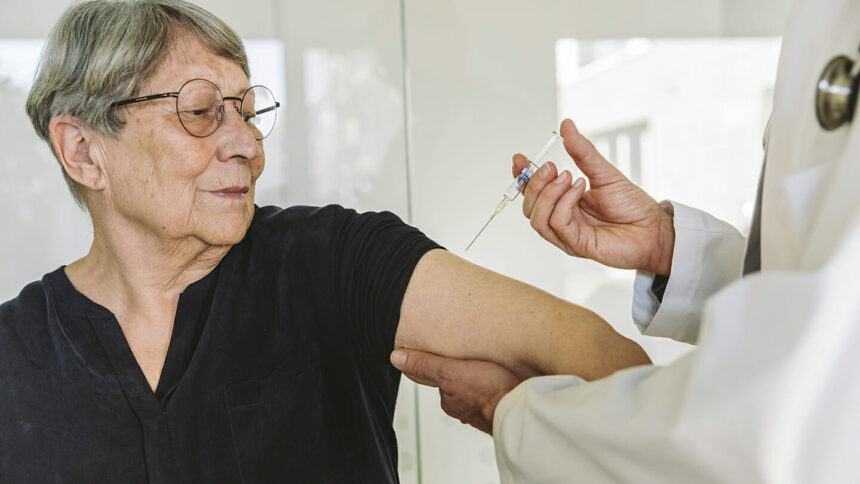
The US Food and Drug Administration (FDA) has approved the messenger RNA-based (mRNA) vaccine against respiratory syncytial virus (RSV) for people ages 60 and older.
The vaccine, made by Moderna, is called mRESVIA. The FDA approved it under an FDA breakthrough therapy designation, which fast-tracks approval. The vaccine should be available for the 2024-2025 RSV season, which starts in the fall. The shot is the only RSV vaccine in a single-dose prefilled syringe, the company pointed out in a press release.
Moderna said there were no serious safety concerns when it tested the shot in people. The most common adverse events were injection-site pain, fatigue and myalgia.
A phase 3 study on more than 37,000 adults, spanning 22 countries, followed people for 3.7 months after receiving the shot. According to the data, the shot showed 83.7% efficacy against lower respiratory tract disease that occurs when people get RSV. At 8.6 months, it was 78.7% effective against lower respiratory tract disease for those who received the shot .
The FDA’s move marks the second Moderna mRNA vaccine to be approved by the FDA. Moderna’s RSV vaccine is the first mRNA vaccine that’s not for COVID-19 and has been approved in the United States.
In other vaccine-related news, about 5 million doses of vaccine against the H5N1 avian flu are being prepared for Americans. The Biomedical Advanced Research and Development Authority (BARDA), which is part of the Administration for Strategic Preparedness and Response (ASPR) and is within the US Department of Health and Human Services (HHS), approved the move.
The bird flu could be mutated and transmitted in humans, according to some medical professionals. CSL Seqirus, which makes the shot, announced last week that it is producing the vaccine in North Carolina.
“It utilizes a highly scalable method of production and is currently positioned to deliver up to 150 million influenza vaccine doses to support an influenza pandemic response within six months of a pandemic declaration,” the company noted in a news release. Moderna is testing an mRNA shot for the bird flu, ABC News reported.




